Sandostatin® (octreotide acetate) Immediate-Release Injection is a medicine approved to treat the severe diarrhea and flushing associated with carcinoid syndrome. It may help you control your symptoms and live in the moment.
Symptom Control You Can Count On
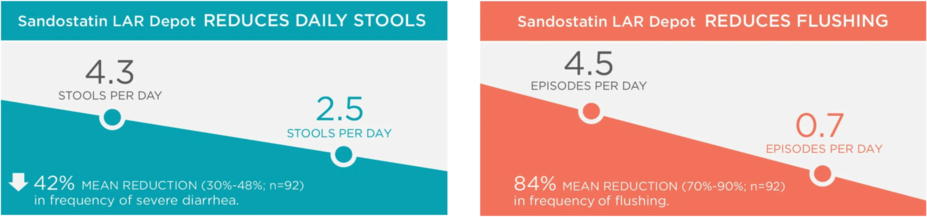
Here are results from a clinical study of 93 patients with carcinoid syndrome who were treated with Sandostatin LAR Depot or Sandostatin Immediate-Release Injection.†
†A 6-month clinical trial of malignant carcinoid syndrome was performed in patients who had previously been shown to be responsive to Sandostatin Immediate-Release Injection. Patients received 10-mg, 20-mg, or 30-mg doses of Sandostatin LAR Depot every 28 days or continued their Sandostatin Immediate-Release Injection regimen. Patients receiving Sandostatin LAR Depot who experienced symptom flare-ups were permitted to use supplemental Sandostatin Immediate-Release Injection until symptoms were again controlled to screening frequency.